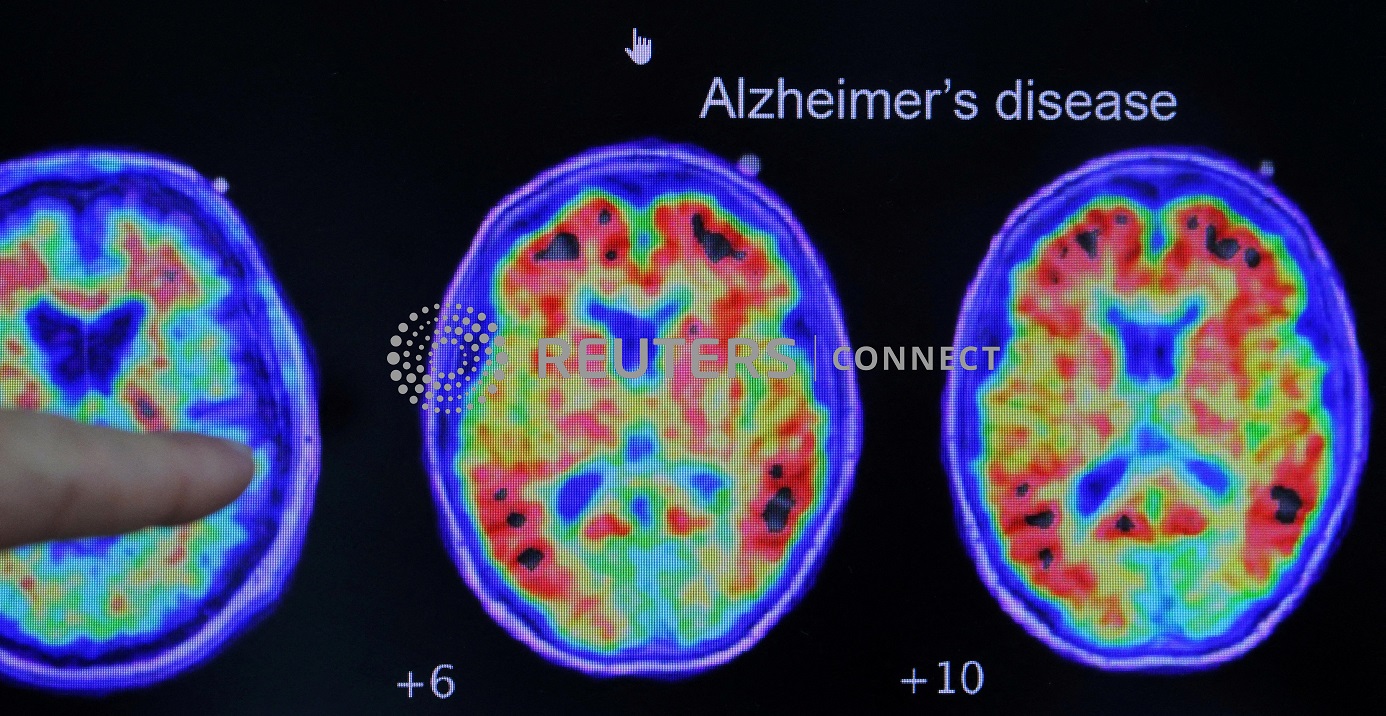
TOKYO (Reuters) – A Japanese health ministry panel on Monday recommended approval of the Alzheimer’s disease treatment Leqembi, following standard approval for the drug granted by U.S. regulators last month.
The expert panel’s decision sets the stage for official approval of the drug, co-developed by Japan’s Eisai and U.S.-based Biogen.
An Eisai executive said this month the company expects to begin marketing Leqembi in Japan within about 60 days of receiving insurance reimbursement approval from the country’s national health system.
(Reporting by Rocky Swift, editing by Ed Osmond)